
Search
Professionals

21-11-11 12:08
Amid on-going Covid-19 global pandemic, interest in Covid-19 treatments has been rapidly growing. Many domestic pharmaceutical companies and research institutes are actively developing treatments and filing patent applications relating to treatment for Covid-19 with Korean Intellectual Property Office (KIPO).
According to KIPO, patent applications relating to Covid-19 treatments have been steadily filed since the outbreak in February last year, and a total of 302 cases were filed by June 2021.
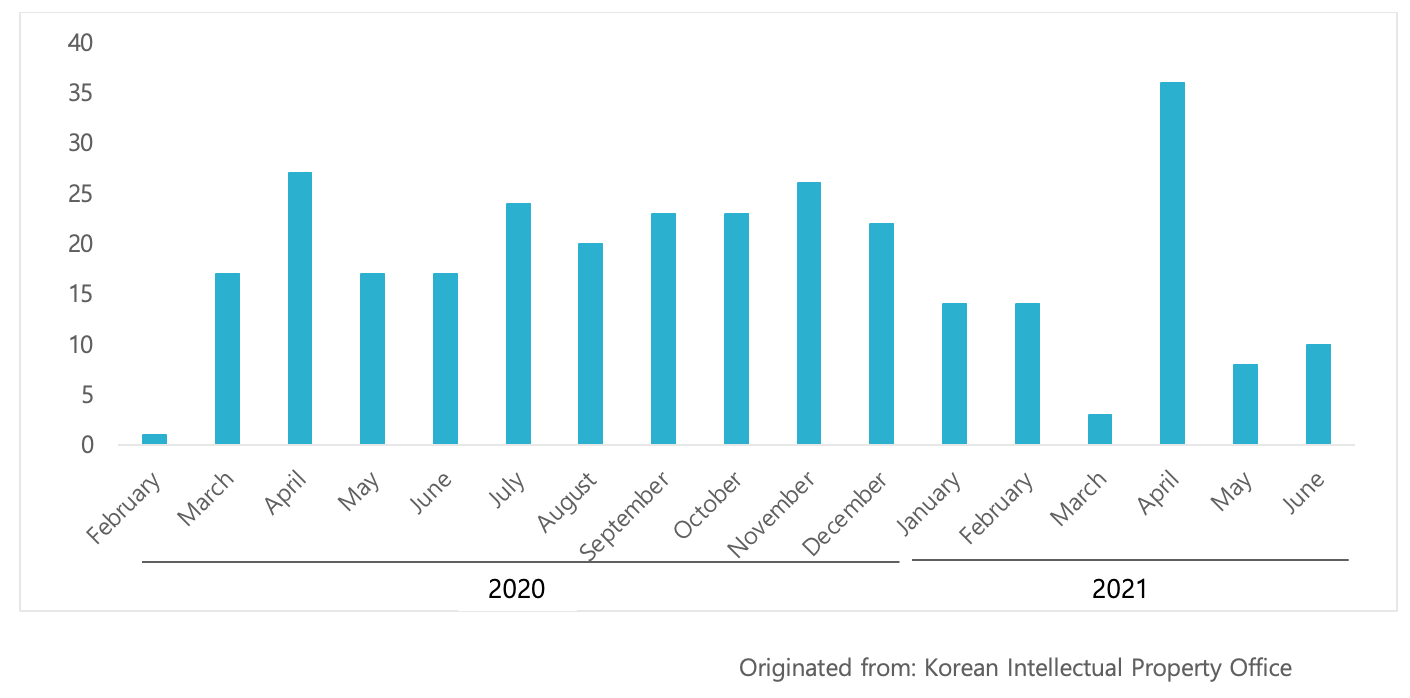
Of those patent applications, thirteen (13) applications were allowed and registered for their antiviral effect on Covid-19, which include Celltrion's Lekkinage, which has been approved as a treatment for Covid-19, Dong-Wha Pharmaceutical's DW2008S (a new drug derived from justicia procumbens), and Bu-Kwang Pharmaceutical's Levovir.
Celltrion's Lekkinage is a monoclonal antibody drug which is selected from B cells of a patient's blood that have been cured after being infected with the Covid-19 (SARS-CoV-2) virus using the phage display method. It is the first treatment for Covid-19 to be approved by the Ministry of Food and Drug Safety as a domestically developed drug. Dong-Wha Pharmaceutical's DW2008S is a natural medicinal product extracted from justicia procumbens. The safety and tolerability of DW2008S was proven in Phase 1 of the clinical trials, which was originally developed for asthma treatment, and it is currently being tested in Phase 2 of the clinical trials for Covid-19 treatment. Bu-Kwang Pharmaceutical's Levovir was originally developed for chronic hepatitis B treatment but it was announced that Phase 2 of the clinical trials for Covid-19 treatment has been completed recently.
In view of patent filings for Covid-19 treatments by applicant, there are 147 cases filed by domestic pharmaceutical companies, 66 cases filed by government agencies and research institutes, 55 cases from universities, 30 cases from individuals, and 4 cases from foreigners. That is, domestic pharmaceutical companies account for a large proportion of 48.7% of the total Covid-19 treatment patent filings, while government agencies and research institutes for 21% and universities for 18%.
To support the development and production of Covid-19 vaccines in Korea, KIPO introduced a pilot program that designates patent applications directed to Covid-19 vaccines to be subject to accelerated examination which will be effective for one year from June 23, 2021. It is expected to see more active filings from related fields for a while until Covid-19 is eradicated.