
Search
Professionals

25-02-21
The revised Pharmaceutical Affairs Act, effective February 21, 2025, eliminates the previous drug re-examination procedures and establishes a clear legal foundation for drug data exclusivity.
Article 31-6 of the revised Pharmaceutical Affairs Act defines data-protected drugs and their protection periods, prohibiting generic or biosimilar companies from applying for a marketing approval by referring to the clinical trial data submitted for the original drug’s marketing approval for a new drug.
In Korea, drug data has been de facto protected under the previous drug re-examination system. However, the revised Pharmaceutical Affairs now clearly introduces drug data exclusivity, ensuring that generic or biosimilar companies cannot immediately rely on the clinical trial data developed by original drug companies, which involved substantial investment of time and resources.
Specifically, generic or biosimilar companies are prohibited from applying for a marketing approval based on the clinical trial data submitted for the original drug’s (“Data-Protected Drug”) approval during the designated protection period (“Data Protection Period”) as set forth below. Exceptions are allowed if (i) the marketing approval holder of the Data-Protected Drug consents to the use of the original drug’s clinical trial data by another company, or (ii) the Ministry of Food and Drug Safety (MFDS) deems it necessary to address a public health crisis. The specific protection criteria, depending on the type and characteristics of the drug, are provided below.
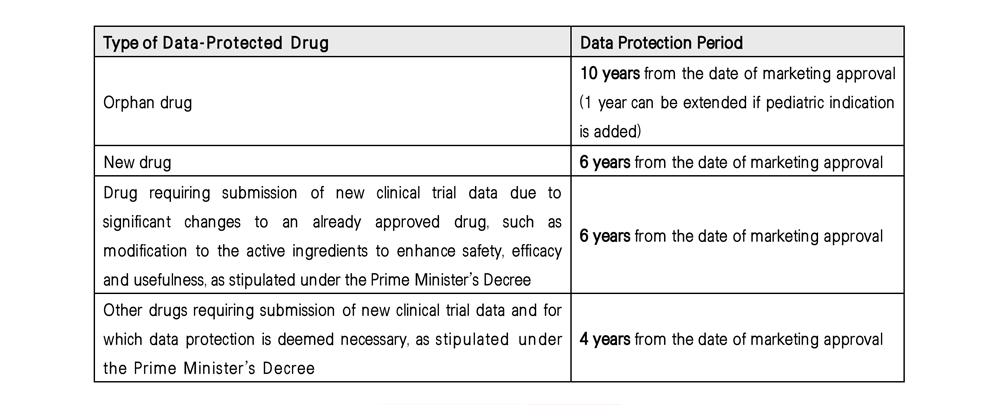
The revised Pharmaceutical Affairs Act is significant in that it clarifies the legal foundation for drug data exclusivity, which was previously protected de facto through the re-examination procedures. It is expected to strengthen market exclusivity for original drug companies, thereby encouraging increased investment in the research and development of new drugs.